We support our customers in transforming their ideas into best-in-class products. Our multidisciplinary R&D team will help you with the following deliverables.
Transforming your ideas into reliable devices
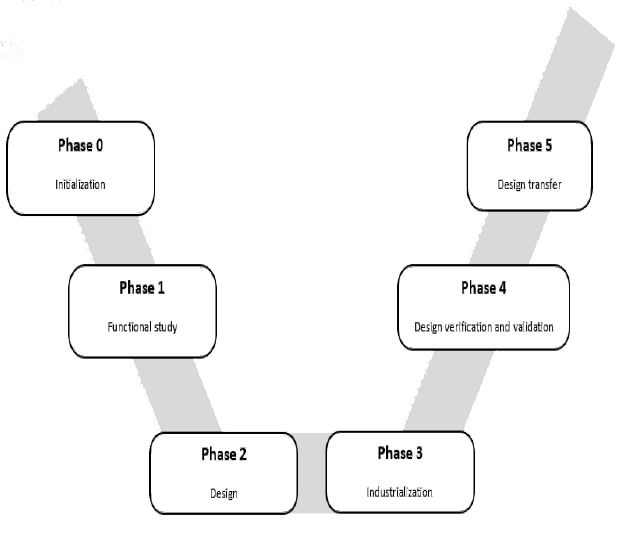
- Project Management: technical specifications, cost and timeframe
- CAD modelling
- Proof of concept
- Tools design and manufacturing
- Prototyping
- V&V activities
- Risk Management activities
- DHFs according to ISO13485 and 21CFR820
- Design transfer activities
- Process validations & Equipment Qualifications (IQ, OQ, PQ)
Our engineering team is dedicated to supporting our customers throughout every stage of their product development journey.
We work as an extension of your internal R&D, providing expertise in:
- Transforming innovative ideas into market-ready products,
- Managing the full development cycle, including design, prototyping, industrialization, manufacturing, and regulatory compliance,
- Ensuring design-for-manufacturing principles are applied from the earliest stages to enable smooth transition from pre-series to full-scale production.
We also design and manufacture custom production tooling and assembly equipment tailored to the specific needs of your device and production volumes. Whether for low-volume pilot runs or high-volume automated manufacturing, our solutions are scalable, robust, and optimized for cost-efficiency and product quality. Our expertise includes the development of injection molds, semi-automated and fully automated assembly stations, and dedicated test fixtures to support validation and production control.
At Circum Life Sciences, we see our R&D team not just as a supplier, but as a true partner, fully integrated into your development process to accelerate innovation and secure successful product launches.